Cancer Cachexia
“Cancer is one example where a small cellular change can alter metabolism so greatly that the body loses its ability to maintain essential nutrients like triglyceride and amino acids. This cachexia syndrome causes patients to waste away and die of frailty and immobility. It has no clear diagnostic methodology, no known mechanism, and no FDA-approved treatment.”
1. Tumor Signals that Drive Cachexia
Despite mice being highly inbred, only 70% of mice with NSCLC develop cachexia. This finding suggests that additional genetic mutations or epigenetic changes arise in the tumors over time and lead to cachexia development. We hypothesize that a tumor-released factor alters systemic organ metabolism, which leads to loss of body weight and skeletal muscle. This “cachexia-inducing factor” has yet to be identified and characterized from endogenous lung tumors. We are using a variety of unbiased strategies to (1) identify proteins that are secreted from the tumor in mice with cachexia, and (2) validating these hits using genetic mouse models.
2. The Liver as a Driver of Cachexia
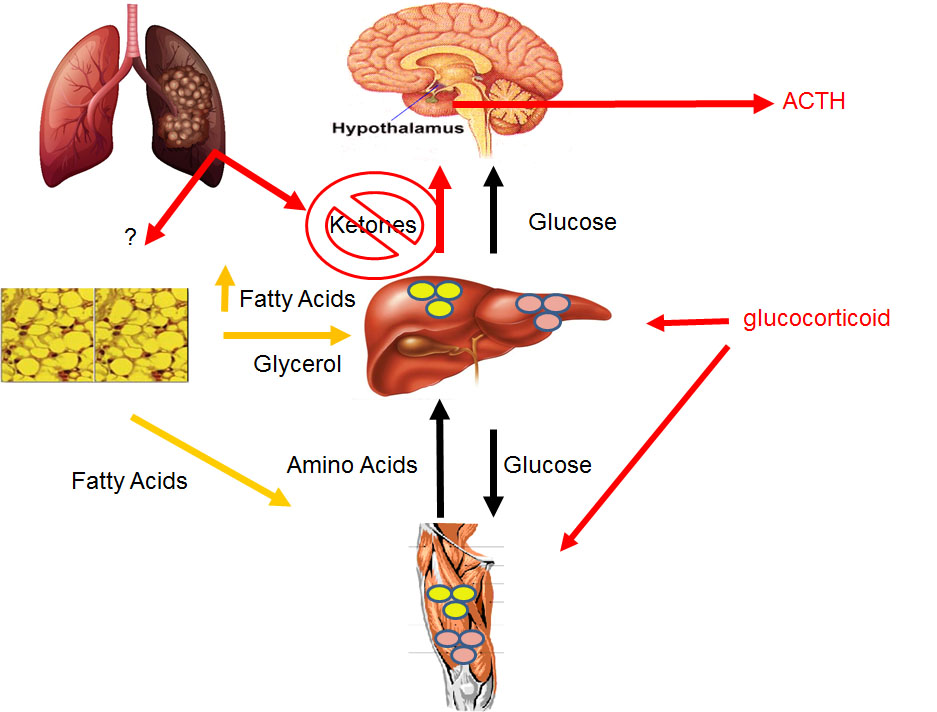
We have identified the loss of hepatic PPARα-dependent ketone production as an important feature of cachexia. Our research program is now focused on how the liver’s metabolism is programmed during tumor progression and how this process leads to cachexia.
3. Adipokines and White Adipose Tissue Dysfunction
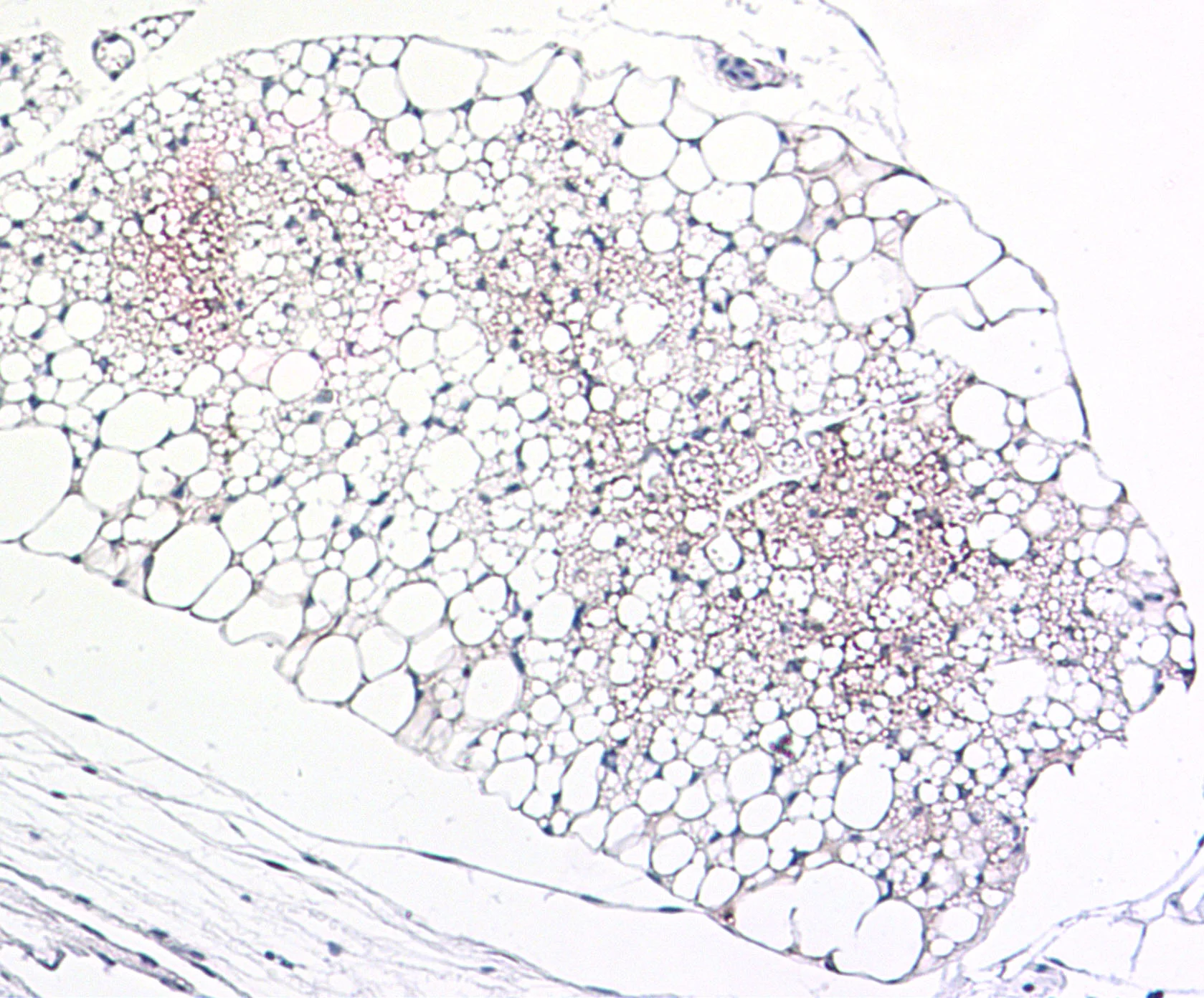
Immunohistochemical (IHC) staining for adiponecting (brown) in adipose tissue
Using molecular and histologic analyses on white adipose tissues from mice with and without cachexia, we found evidence of widespread dysfunction including a lipodystrophy-like phenotype. For example, leptin and adiponectin are severely reduced in the tissue and plasma. This research program is investigating strategies to improve the health of adipose tissue as a therapeutic strategy to prevent muscle wasting.
4. Sex-dependent Combination Therapies
Our lab has developed novel combination therapies that reverse cachexia and prolong survival in mice with lung cancer. Surprisingly, these combinations work in a sex-dependent manner. Female mice respond to TGF-b family inhibitors and male mice response to IL-6 superfamily inhibitors. We are now investigating the mechanisms underlying these responses.
Active Grants:
Cancer Research UK – National Cancer Institute Cancer Grand Challenge, CANcer Cachexia Action Network/CANCAN. Co-PI, 03/01/2022 – 02/28/2027. £1,700,000.
Prior Grants:
NCI Mentored Clinical Scientist Research Career Development Award to Promote Diversity (K08), The Role of Hypoketonemia in the Cancer Anorexia-Cachexia Syndrome, 1K08CA230318, PI, 08/01/18-07/31/23. $598,517
Lung Cancer Research Foundation, Weill Cornell Medical College, Molecular Mechanisms of Cachexia in Non-Small Cell Lung Cancer, PI, 11/01/17-10/31/19. $150,000
Pfizer Collaborative Research Agreement, Identifying Novel Modulators of the Cancer Anorexia Cachexia Syndrome, PI, 04/01/19 – 09/30/19, $40,318
Myos Rens Technology, To Test the Efficacy of Fortetropin to Prevent Muscle Wasting in a Mouse Model of Lung Cancer, PI, 05/01/18-12/31/18. $45,691